Question 1.
What would be the final temperature of a mixture of 50 g of water at 20° C temperature and 50 g of water at 40° C temperature? (AS1)
Answer:
In CGS system :
Mass m1= 50 g
Higher temperature = T1= 40° C
Mass m2= 50 g
Lower temperature = T2= 20° C
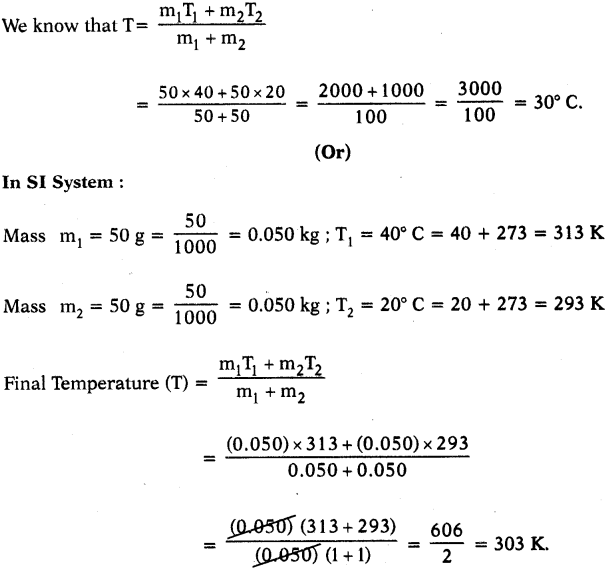
Question 2.
Explain, why dogs pant during hot summer days using the concept of evaporation. (AS1)
(OR)
How do dogs cool their body? Explain by using the process of evaporation.
Answer:
- Dogs pant during hot summer days and get their body cooled. This cooling effect is due to evaporation.
- Evaporation is a surface phenomenon. Temperature of a system falls during evaporation.
- During summer the temperature in the human body increases.
- The temperature of the skin becomes higher and the water in the sweat glands starts evaporating. Since evaporation is a cooling process human body becomes cool.
- Dogs don’t have sweat glands. Their body is covered with hair. They have sweat glands only in their feet.
- So by panting the water on the tongue undergoes evaporation resulting in the cooling of the dog’s body.
Question 3.
Why do we get dew on the surface of a cold soft drink bottle kept in open air? (AS1)
(OR)
Raju observed small droplets of water outside a cold soft drink bottle kept in open air. What is the reason for the formation of droplets?
Answer:
- When cold soft drink bottle is kept in open air, the temperature of surrounding air is higher than the temperature of cold drink bottle.
- Air contains molecules in the form of vapour.
- During the motion of water molecules in air strike the surface of cold drink bottle.
- Then the molecules of air lose their kinetic energy which leads to lower the temperature and they convert into droplets.
- So dew is formed on the surface of cold soft drink bottle.
Question 4.
Write the differences between evaporation and boiling. (AS1)
(OR)
Sita observed decrease in quantity of spirit kept in a vessel placed in open air. Whereas Ramu observed formation of bubbles on a water surface when it is heated. What are those two processes? Distinguish between those two processes.
Answer:
| Evaporation |
Boiling |
| 1. The process of escaping of molecules from the surface of a liquid at any temperature is called evaporation. |
1. The process in which the liquid phase changes to gaseous phase at constant temperature. This temperature is called boiling point of liquid. |
| 2. Evaporation takes place at any temperature. |
2. Boiling takes place at a definite temperature. |
| 3. The temperature of liquid gets down. |
3. The temperature of liquids increases up to a constant temperature. |
| 4. The kinetic energy does not change. |
4. The kinetic energy of the molecules increases with the increase of temperature. |
| 5. The evaporation depends on surface area, wind, speed, humidity. |
5. The boiling depends on atmospheric pressure. |
| 6. It is surface phenomenon. |
6. It is bulk phenomenon. |
7. Eg : 1) Wet clothes dries.
2) Sea water evaporates to form clouds. |
7. Eg : 1) Water boils at 100° C. |
Question 5.
Does the surrounding air become warm or cool when vapour phase of H2O condenses? Explain. (AS1)
Answer:
- Gases have more higher energy than liquids and solids.
- When vapour condenses, it changes from gas to liquid.
- Therefore there is a drop in energy.
- This energy has to go (somewhere) to the surroundings.
- So surrounding air becomes warm when vapour phase of H20 condenses.
Question 6.
Answer these. (AS1)
a) How much energy is transferred when 1 gm of boiling water at 100°C condenses to water at 100°C?
Answer:
CGS system :
Mass of water = m = 1 gm
Latent heat of vapourisation = 540 cal/gm.
The amount of heat energy released when 1 gm of boiling water at 100°C condenses to water at 100°C
Q = mLvapour= 1 × 540 = 540 cal.
(OR)
In SI system :
Mass of water = m = 1 gm = 1 × 10-3kg
Latent heat of vapourisation = 540 cal/gm.
The amount of heat energy released when 1 gm of boiling water at 100°C condenses to water at 100°C
Q = mLvapour- 1 × 540 = 540 cal.
In SI, Q = 540 × 4.18 = 2257 J.
b) How much energy is transferred when 1 gm of boiling water at 100° C cools to water 0° C?
Answer:
CGS system :
Latent heat of vapourisation = 540 cal/gm
The amount of heat energy released when 1 gm of boiling water at 100°C condenses to water at 100 °C. m = 1 gm.
Q1= mLvapour= 1 × 540 = 540 cal.
The specific heat of water = 1 cal/gm-°C
Difference in temperature = 100-0 = 100°C.
The heat released to cool water to 0°C is
Q2= mS∆T = 1 × 1 × 100 = 100 cal.
∴ Total energy released = 540 + 100 = 640 cal.
c) How much energy is released or absorbed when 1 gm of water at 0° C freezes to ice at 0° C?
Answer:
In CGS system :
Mass of water = m = 1 gm
Latent heat of fusion of ice (L) = 80 cal/gm
The energy transferred or released when 1 gm of water at 0° C freezes to ice at 0° C.
Q = mLfreeze= 1 × 80 = 80 Cal.
(OR)
In SI system :
Mass of water = m = 1 gm =1/1000kg
Latent heat of fusion = L = 3.36 × 105J/kg.
Amount of heat released or transferred when lgm of water at 0°C freezes to ice at 0°C.
Q = mLfusion=1/1000kg× 3.36 × 105= 3.36 × 102= 336J.
(OR)
In CGS system :
Mass of water = m = 1 gm
Latent heat of fusion of ice (L) = 80 cal/gm
The energy transferred or released when 1 gm of water at 0° C freezes to ice at 0°C.
Q = mLfreeze= 1 × 80 - 80 cal.
(Or)
In SI system : In SI, Q = 80 × 4.2 [1 cal = 4.2 J]
Q = 1 x 10-3× 3.36 × 105= 3 36 J.
d) How much energy is released or absorbed when 1 gm of steam at 100°C turns to ice at 0°C?
Answer:
In CGS system :
Mass of water = m = 1 gm
Latent heat of vapourisation = Lvapour= 540 cal/gm
Latent heat of fusion of ice = Lfusion= 80 cal
Specific heat of water = S = 1 cal/gm-0°C
Difference in temperature
∆T = 100 - 0 = 100°C.
The energy transferred when 1 gram of steam at 100°C turns to ice at 0°C
Q = mLvapour+ mS∆T + mLfusion
= 1 × 540 + 1 × 1 × 100 + 1 × 80 = 540 + 100 + 80 = 720 cal.
(OR)
Mass of water = m = 1 gm =1/1000kg
Latent heat of vapourisation = Lvapour= 2.25 × 106J/kg
Latent heat of fusion = Lfusion= 3.36 × 105J/kg
Difference in temperature
∆T = 373 - 273 - 100 K.
Specific heat of water = 4180 J/kg-K
The energy transferred when 1 gram of steam at 100° C turns to ice at 0°C =
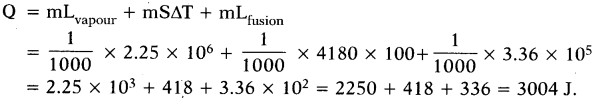
(OR)
In CGS system :
Conversion : Steam at 100°C → Water at 100°C → Water at 0°C → Ice at 0°C.
Mass of water = m = 1 gm
Latent heat of vapourisation = Lvapour= 540 cal/gm
Latent heat of fusion of ice = Lfusion= 80 cal
Specific heat of water = S = 1 cal/gm-0°C
Difference in temperature
∆T= 100-0 = 100°C.
The energy transferred when 1 gram of steam at 100°C turns to ice at 0°C
Q = mLvapour+ mS∆T + mLfusion
= 1 × 540 + 1 × 1 × 100 + 1 × 80
= 540 + 100 + 80 = 720 cal. = 720 × 4.18 = 3009.6 J.
Question 7.
Explain the procedure of finding specific heat of solid experimentally. (AS1)
(OR)
Determination of specific heat of solid experimentally.
(OR)
Ravi wanted to prepare solid with high specific heat to use on cooking utensil. What fool does he need to find the specific heat of aluminium and copper? How should he conduct the experiment?
Answer:
Aim : To find the specific heat of given solid.
Apparatus : Calorimeter, thermometer, stirrer, water, steam heater, wooden box and lead shots.
Procedure:
- Measure the mass of the calorimeter with stirrer = m1gm
- Fill water one third volume of calorimeter and measure the mass = m2gm.
- At this time initial temperature = T1.
- Mass of the water = m2- m1gm.
- Take a few lead shots and place them in steam heater and heat up to 100° C. Let this temperature be T2.
- Transfer the lead shots into calorimeter and measure the final (or) resultant temperature T3.
- Mass of calorimeter with contents = m3gm and mass of lead shots = m3- m2gm.
- If the specific heats of the calorimeter, lead shots and water are Sc, Sland Swrespectively, by using method of mixtures we have
Heat lost by the solid = Heat gained by the calorimeter + water
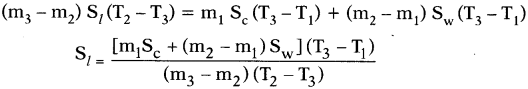
Knowing the specific heats of calorimeter and water we can calculate specific heat of solid (lead shots).
Question 8.
Convert 20° C into Kelvin scale. (or) Change 20°C into absolute scale. (AS1)
Answer:
T = t°C + 273 = 20 + 273 = 293
⇒ T = 293 K.
Question 9.
Your friend is asked to differentiate between evaporation and boiling. What questions could you ask to make him to know the differences between evaporation and boiling? (AS2)
(OR)
Veena found that the water kept in a pot is cool and Siva observed when water is heated the temperature remains constant for some time until water turns into vapour. What are the processes involved in these two aspects? Ask some questions to understand these aspects.
Answer:
The questions asked by me are :
- How do wet clothes get dried without heating?
- Are boiling and evaporation one and same or different?
- Is there any difference in kinetic energy if it boils?
- Is the temperature the main cause for boiling and evaporation?
- What are the factors which influence evaporation?
- Is boiling temperature for water always 100° C?
Question 10.
What happens to the water when wet clothes dry? (AS3)
Answer:
- When wet clothes dry, the water present in the clothes is evaporated.
- So that the process of evaporation causes the wet clothes dry.
Question 11.
Equal amounts of water are kept in a cap and in a dish. Which will evaporate faster? Why? (AS3)
(OR)
Srinu kept. equal amounts of water in a cap and in a dish in open air. What is his observation? Explain the experiment.
Answer:
Aim : To show the evaporation of equal amounts of water in cap and dish.
Apparatus : Cap, dish, water.
Procedure :
- Take equal amounts of water in cap and dish. Keep them in open air for two hours. Now weigh the water in the cap and the dish.
- We can observe that the weight of water in dish is less than that of water in cap.
- This shows that the water in dish has more evaporation than the water in cap.
- It is due to more surface area of dish.
- As the surface area increases rate of evaporation also increases.
Question 12.
Suggest an experiment to prove that rate of evaporation of a liquid depends on its surface area and vapour already present in surrounding air. (AS3)
Answer:
Aim: The rate of evaporation of liquid depends on its surface area and vapour already present in surrounding air.
Apparatus : Two dishes of different surface areas and water.
Procedure :
- Take two dishes of different surface area.
- Pour equal amounts of water in the both dishes.
- Keep aside for two to three hours.
- Observe them after some time.
Dish with more surface area has less quantity of water than the dish having less surface area. ,
- This shows evaporation increases with increasing of surface area.
- Take two dishes of equal surface area containing water.
- This experiment should be conducted on more humid day and less humid day.
- We will find that evaporation is less on more humid day due to more vapour in the air.
- So evaporation decreases with vapour in the air.
Question 13.
Place a Pyrex funnel with its mouth-down in a sauce pan full of water, in such a way that the stem tube of the funnel is above the water or pointing upward into air. Rest the edge of the bottom portion of the funnel on a nail or on a coin so that water can get under it. Place the pan on a stove and heat it till it begins to boil. Where do the bubbles form first? Why? Can you explain how a natural geyser works using this experience? (AS4)
Answer:
- When Pyrex funnel with its mouth down in a sauce pan then the bubbles formed by the heat energy come from the top of the funnel.
- That is from stem tube.
- This is because of pressure inside mouth of funnel increases rapidly due to increasing of heat energy.
- Pressure inside the funnel rs more than outside the funnel and very high at stem.
- Hence, bubbles come from stem of the funnel and escapes through stem tube with force, like a geyser.
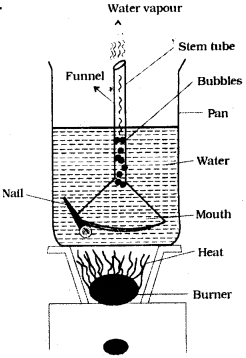
Working of natural geyser by using this experience :
- Geysers are the fountains of hot water coming under the layers of the earth.
- It is a hole with narrow and deep from the bottom of the earth layers.
- It contains water.
- Water heats up due to high temperatures of the inner layers of the earth.
- As by the pressure of water at top layers of the hole, temperature rises, water boils.
- This hot water comes with narrow vent with high pressure, like Lava from the Volcano.
Question 14.
Collect the information about working of natural geyser and prepare a report. (AS4)
Answer:
Natural Geysers :
- Geysers are the fountains of hot water coming under the layers of the earth.
- It is a hole with narrow and deep from the bottom of the earth layers.
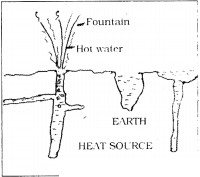
- It contains water.
- Water heats up due to high temperatures of the inner layers of the earth.
- As by the pressure of water at top layers of the hole, temperature rises, water boils.
- This hot water comes with narrow vent with high pressure, like Lava from the Volcano.
- This looks like a water fountain at the surface of the earth.

Question 15.
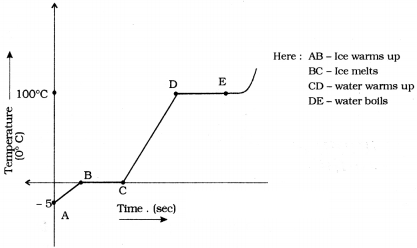
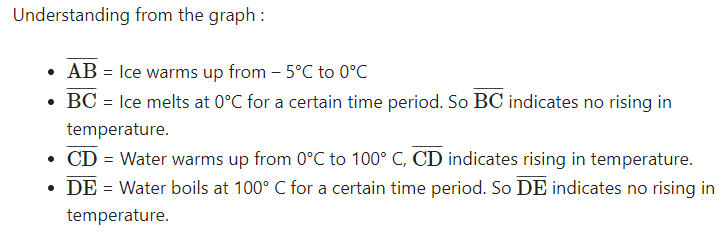
Assume that heat is being supplied continuously to 2 kg of ice at - 5°C. You know that ice melts at 0°C and boils at 100°C. Continue the heating till it starts boiling. Note the temperature for every minute. Draw a graph between temperature and time using the values you get. What do you understand from the graph ? Write the conclusions. (AS5)
Answer:
Graph between time and temperature from ice melting at 5° C to boils at 100° C.
Conclusion :
- The temperature remains same at 0° C until all the ice converted into water. So, 0° C is the melting point of water.
- The temperature remains constant at 100° C until all the water converted into water vapour. So, 100° C is the boiling point of the water.
Question 16.
How do you appreciate the role of the higher specific heat of water in stabilising atmospheric temperature during winter and summer seasons? (AS6)
Answer:
- Due to higher specific heat of water oceans absorb the solar energy for maintaining a relatively constant temperature.
- Oceans absorb large amounts of heat at the equator.
- The oceans moderate the surrounding temperature near the equator.
- Ocean water transports the heat away from the equator to areas closer to the north and south pole.
- This transported heat helps moderate the climate in parts of the Earth that are far from the equator.
- So higher specific heat of water is stabilising atmospheric temperature.
- So we have to extremely appreciate the role of higher specific heat of water to stabilise the atmospheric temperature.
Question 17.
Suppose that 1 / of water is heated for a certain time to rise and its temperature by 2°C. If 2 l of water is heated for the same time, by how much will its temperature. (AS7)
Answer:
Mass of 1 litre of water (m1) = 1 kg ; ∆T1= 2°C
Mass of 2 litres of water (m2) = 2 kg ; ∆ T2= ?
Time duration is same. So same heat is absorbed by water in both the cases
⇒ Q1= Q2
m1S(∆T1) = m2S (∆T2)
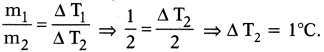
So the rise in temperature for 2 kg of water = 1°C.
Question 18.
What role does specific heat play in keeping a watermelon cool for a long time after removing it from a fridge on a hot day? (AS7)
Answer:
- Generally, watermelon contains large percentage of water.
- Water has high specific heat value than other substances.
- High specific heat substances oppose the increase of temperature. Hence they continuous of the coolingness.
- So watermelon retains coolness after removing from fridge on a hot day due to the high specific cheat of water.
Question 19.
If you are chilly outside the shower stall, why do you feel warm after the bath if you stay in bathroom? (AS7)
Answer:
- In the bathroom, the number of vapour molecules per unit volume is greater than the number of vapour molecules per unit volume outside the room.
- When we try to dry ourselves with a towel, the vapour molecules surrounding you condense on your skin.
- Condensation is a warming process.
- Because of the condensation, you feel warm outside the shower stall when it is chilly.
Question 20.
Three objects A at 30°C, B at 303K and C at 420 K are in thermal contact. Then answer the follwing questions.
(i) Which are in “Thermal equibrium” among A, B and C?
(ii) From which object to another heat transferred? (2 Marks)
Answer:
i) 303K – 273K + 30K = 0°C + 30°C = 30°C.
∴ A and B objects are in ‘Thermal equibrium”.
ii) From object ‘C’ to objects ‘A’ and ‘B’ heat transferred.
Fill in the Blanks
1. The SI unit of specific heat is …………………. .
2. …………………. flows from a body at higher temperature to a body at lower temperature.
3. …………………. is a cooling process.
4. An object A at 10° C and another object B at 10 K are kept in contact, then heat will flow from …………………. to …………………. .
5. The latent heat of fusion of ice is …………………. .
6. Temperature of a body is directly proportional to …………………. .
7. According to the principle of method of mixtures, the net heat lost by the hot bodies is equal to …………………. by the cold bodies.
8. The sultryness in summer days is due to
9. …………………. is used as a coolant.
10. Ice floats on water because …………………. .
Answer:
- J/kg - K
- Heat
- Evaporation
- A, B
- 80 cal/gm
- Average kinetic energy of the molecules of the body.
- net heat gained
- high humidity
- Water
- the density of ice is less than that of water