NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements Important Questions with Answers
Short Answer Type Questions
Question1.
The three elements A, B and C with similar properties have atomic masses X, Y and Z, respectively. The mass of Y is approximately equal to the average mass of X and Z. What is such an arrangement of elements called? Give one example of such a set of elements?
Year of Question :(2014 D)
Answer:
Dobereiner proposed a Triad of elements. He arranged elements in triads. In it, the atomic mass of the middle element is equal to the average atomic mass of the other two elements. Such an arrangement of elements is called a triad.
For example, the Atomic mass of Lithium, Sodium and Potassium are 6.9, 23.0 and 39.0, respectively. The average mass of Li and K is approximately 23.0, equal to the atomic mass of Na. Thus, Lithium, Sodium and Potassium make a triad
Question2.
Elements have been arranged in the following sequence based on their increasing atomic masses.
F, Na, Mg, AI, Si, P, S, CI, Ar, K?
Year of Question :(2014 D)
- (a) Pick two sets of elements with similar properties
- (b) The given sequence represents which law of classification of elements
Answer:
- (a) (i) F and Cl (ii) Na and K have similar properties
- (b) It represents Newlands law of octaves
Question3.
Can the following groups of elements be classified as Dobereiners triad?
Year of Question :(2014 )
- (a) Na, Si, CI
- (b) Be, Mg, Ca
Atomic mass of Be 9; Na 23; Mg 24; Si 28; C| 35; Ca 40
Explain by giving a suitable reason
Answer:
- (a) Na, Si, and Cl are not a Dobereiners triad. Although the atomic mass of silicon (Si) is the average of atomic masses of sodium (Na) and chlorine (Cl), but these elements do not possess similar properties. Hence, it cant be classified as a Dobereiners triad.
23 (Na) + 35 (Cl) / 2 = 29 (Si)
- (b) Be, Mg, and Ca is a Dobereiners triad. They have similar properties, and the atomic masses of magnesium (Mg) is approximately the average of the atomic mass of Be and Ca.
9 (Be) + 40 (Ca) / 2 = 24.5 (Si)
Question4.
In Mendeleevs Periodic Table, the elerents were arranged in the increasing order of their atomic masses. However, cobalt with an atomic mass of 58.93 amu was placed before nickel, having an atomic mass of 58.71 amu. Give a reason for the same?
Year of Question :(2014)
Answer:
In Mendeleevs Periodic Table, the elements were arranged in the increasing order of their atomic masses. However, cobalt with an atomic mass of 58.93 amu was placed before nickel, having an atomic mass of 58.71 amu. We did this to ensure that the same group has elements with similar properties. Hence cobalt was placed before nickel despite the higher atomic number of Cobalt than Nickel
Question5.
Hydrogen occupies a unique position in Modern Periodic Table". Justify the statement?
Year of Question :(2014)
Answer:
Hydrogen has an electron configuration of 1s1. It can either accept one electron to get a stable configuration or donate one electron to become stable. Hence the position of hydrogen isnt evident. We can place it either in the metals group as it donates or in the nonmetal group as it accepts an electron. Hence it occupies a unique position in the periodic table
Question6.
Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev?
Year of Question :(2013)
Answer:
The formulae of chlorides of Eka-silicon and Eka-aluminium are XC4 and XCl3, respectively.
Question7.
Three elements A, B and C have 3, 4 and 2 electrons, respectively, in their outermost shell. Give the group number to which they belong in the Modern Periodic Table. Also, give their valencies?
Year of Question :(2013)
Answer:
A belongs to Group 13, B belongs to Group 14, and C belongs to Group 2. A valency is 3, B valency is 4, and C valency is 2.
Question8.
If an element X is placed in group 14, what will be the formula and the nature of bonding
of its chloride?
Year of Question :(2012)
Answer:
If an element is placed in group 14, it has 4 electrons in its outermost orbit. Hence, the formula of its chloride is XCl4. It makes compounds by sharing electrons, so its compound will have a covalent bond
Question9.
Compare the radii of two species, X and Y. Give reasons for your answer?
Year of Question :(2013)
- (a) X has 12 protons and 12 electrons
- (b) Y has 12 protons and 10 electrons
Answer:
As both X and Y had 12 protons, their atomic number is 12, i.e., magnesium. Y has only 10 electrons, due to which the effect of nuclear charge on the electrons would be more than that of 12 electrons. Hence, the radii of Y would be smaller than that of X
Question10.
Arrange the following elements in increasing order of their atomic radii?
Year of Question :(2013)
- (a) Li, Be, F, N
- (b) CI, At, Br, I
Answer:
- (a) Li, Be, N, and F are in the same period, and atomic radii decrease from left to right. Thus, the order will be: F < N < Be < Li
- (b) Cl, Br, I, and At are in the same group, and the atomic radii increase from top to bottom. Thus, the order will be: CI < Br < I < At
Question11.
Identify and name the metals from the following elements whose electronic configurations are given below?
Year of Question :(2013)
- (a) 2, 8, 2
- (b) 2, 8, 1
- (c) 2, 8,7
- (d) 2, 1
Answer:
- (a) Magnesium; It is a metal
- (b) Sodium; It is a metal
- (c) Chlorine; It is a nonmetal
- (d) Lithium; It is a metal
Question12.
Write the formula of the product formed when element A (atomic number 19) combines with element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
Year of Question :(2012)
Answer:
The products formula when element A (atomic number 19) combines with element B (atomic number 17) is KCl.
The nature of the bond between KCl is ionic.
Electron Dot Structure of KCl

Question13.
Arrange the following elements in the increasing order of their metallic character: Mg, Ca, K, Ge, Ga?
Year of Question :(2013)
Answer:
Metallic character increases as we move down the group because there is an increase in atomic size.
Thus, the order will be: Ge< Ga < Mg < Ca < K
Question14.
Identify the elements with the following property and arrange them in increasing order of their reactivity?
Year of Question :(2013)
- (a) An element which is a soft and reactive metal
- (b) The metal which is an important constituent of limestone
- (c) The metal which exists in a liquid state at room temperature
Answer:
- (a) Sodium is soft and reactive
- (b) Calcium is an important constituent of limestone
- (c) Mercury exists in a liquid state at room temperature
The increasing order of reactivity will be: Hg < Ca < Na.
Question15.
Properties of the elements are given below. Where would you locate the following elements in the periodic table?
Year of Question :(2013)
- (a) A soft metal stored under kerosene
- (b) An element with variable (more than one) valency stored underwater
- (c) An element which is tetravalent and forms the basis of organic chemistry
- (d) An element which is an inert gas with atomic number 2.
- (e) An element whose thin oxide layer is used to make other elements corrosion-resistant by anodising
Answer:
- (a) Sodium is soft metal stored under kerosene.
- (b) Phosphorous shows variable (more than one) valency and is stored underwater
- (c) Carbon is a tetravalent element and forms the basis of organic chemistry
- (d) Helium is an element which is an inert gas with atomic number 2
- (e) Aluminium is an element whose thin oxide layer is used to make other elements corrosion-resistant by anodising
Long Answer Type Questions
Question1.
An element is placed in the 2nd Group and 3rd Period of the Periodic Table. It burns in the presence of oxygen to form a basic oxide?
Year of Question :(2010)
- (a) Identify the element
- (b) Write the electronic configuration
- (c) Write the balanced equation when it burns in the presence of air
- (d) Write a balanced equation when this oxide is dissolved in water
- (e) Draw the electron dot structure for the formation of this oxide
Answer:
- (a) Magnesium
- (b) The electronic configuration of magnesium is 2, 8, 2
- (c ) 2 Mg + O2→ 2 MgO
- (d) MgO + H2O → Mg(OH)2
- (e) Electron Dot Structure of magnesium oxide
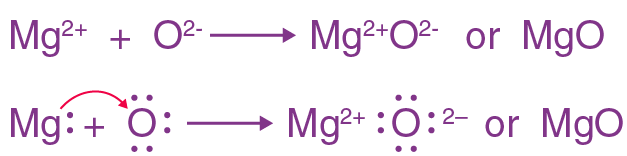
Question2.
An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide?
Year of Question :(2011)
- (a) Where in the periodic table are elements X and Y placed
- (b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
- (c) What will be the nature of the oxide of element Y? Identify the nature of bonding in the compound formed
- (d) Draw the electron dot structure of the divalent halide
Answer:
- (a) X belongs to Group 17 and 3rd periods, while Y belongs to Group 2 and 4th
- (b) X is a Nonmetal, while Y is a metal
- (c) Y will form a basic oxide, and it will have an ionic bondingv
- (d) Electron dot structure of the divalent halide
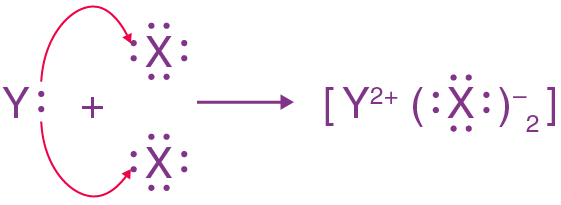
Question3.
The atomic number of a few elements are given below
10, 20, 7, 14?
Year of Question :(2012)
- (a) Identify the elements
- (b) Identify the Group number of these elements in the Periodic Table
- (c) Identify the Periods of these elements in the Periodic Table
- (d) What would be the electronic configuration for each of these elements
- (e) Determine the valency of these elements
Answer:
- (a) 10 is the atomic number of neon.
20 is the atomic number of calcium
7 is the atomic number of nitrogen.
14 is the atomic number of silicon.
- (b) Neon belongs to group 18.
Calcium belongs to group 2.
Nitrogen belongs to group 15.
Silicon belongs to group 14
- (c ) Neon belongs to period 2.
Calcium belongs to period 4.
Nitrogen belongs to period 2.
Silicon belongs to period 3
- (d) The electronic configuration of neon is 2, 8.
The electronic configuration of calcium is 2, 8, 8, 2.
The electronic configuration of nitrogen is 2, 5.
The electronic configuration of silicon is 2, 8, 4
- (e) The valency of neon is equivalent to 8 - no of valence electrons, i.e. 8 - 8 = 0.
The valency of calcium is equivalent to the no of valence electrons, i.e. equal to 2.
The valency of nitrogen is equivalent to 8 - no of valence electrons, i.e. 8 - 5 = 3.
The valency of silicon is equivalent to 8 - no of valence electrons, i.e. 8 - 4 = 4
Question4.
Complete the following crossword puzzle (Figure 5.1)?
Year of Question :(2013)
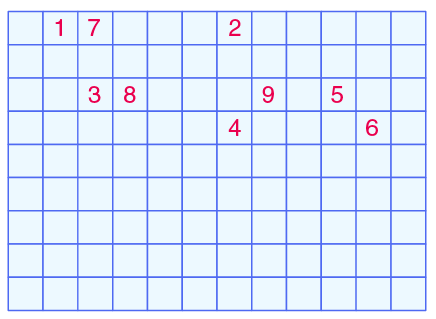
- (1) An element with atomic number 12
- (3) Metal used in making cans and member of Group 14
- (4) A lustrous non-metal with 7 electrons in its outermost shell.
Down:
- (2) Highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene.
- (5) The first element of the second Period
- (6) An element which is used in making fluorescent bulbs and is the second member of Group 18 in the Modem Periodic Table
- (7) A radioactive element which is the last member of the halogen family
- (8) Metal is an important constituent of steel and forms rust when exposed to moist air
- (9) The first metalloid in Modem Periodic Table whose fibres are used to make bullet-proof vests
Answer:
- (1) Magnesium has 12 atomic numbers
- (2) Sodium is a highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene
- (3) Tin is used in making cans and is a member of Group 14
- (4) Iodine is a lustrous non-metal with 7 electrons in its outermost shell
- (5) Lithium is the first element of the second Period
- (6) Neon is used in making fluorescent bulbs and is the second member of Group 18 in the Modem Periodic Table
- (7) Astatine is a radioactive element which is the last member of the halogen family
- (8) Iron is an important constituent of steel and forms rust when exposed to moist air
- (9) Boron is the first metalloid in Modem Periodic Table whose fibres are used to make bullet-proof vests
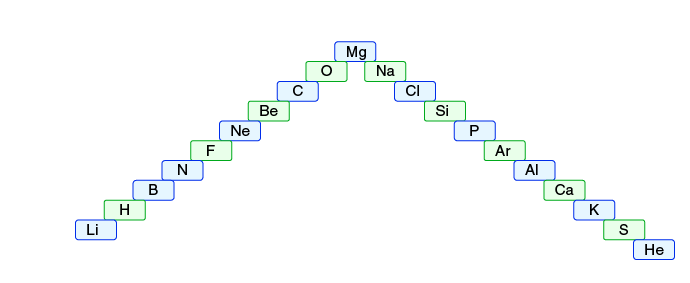
Question5.
- (a) In this ladder (Figure 5.2), symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table
- (b) Arrange them in the order of their group also
Answer:
- (a) The arrangement of elements in the increasing order of their atomic number in the Periodic Table.
H, He, Li, Be, B, C, N, O, F, Ne, Na, Mg, Al, Si, P, S, CI, Ar, K, Ca
- (b) The arrangement of elements in groups.
Group 1: H, Li, Na, K
Group 2: Be, Mg, Ca
Group 13: B, Al
Group 14: C, Si
Group 15: N, P
Group 16: O, S
Group 17: F. Cl
Group 18: He, Ne, Ar
Question6.
Mendeleev predicted the existence of certain elements not known at that time and named two of them Eka-silicon and Eka-aluminium?
Year of Question :(2014)
- (a) Name the elements which have taken the place of these elements
- (b) Mention the group and the period of these elements in the Modern Periodic Table
- (c) Classify these elements as metals, non-metals or metalloids
- (d) How many valence electrons are present in each of them
Answer:
- (a) Eka-silicon was replaced by Germanium, while Gallium replaced Eka-aluminium
- (b) Gallium belongs to Group 13 and Period 5 of the periodic table, while Germanium belongs to Group 14 and Period 4
- (c) Gallium is metal, while Germanium is metalloid
- (d) Gallium has three valence electrons, while Germanium has 4
Question7. ?
- a) Electropositive nature of the element(s) increases down the group and decreases across the period
- (b) Electronegativity of the element decreases down the group and increases across the period
- (c) Atomic size increases down the group and decreases across a period (left to right)
- (d) Metallic character increases down the group and decreases across a period.
Based on the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
- (a) Name the most electropositive element among them
- (b) Name the most electronegative element among them
- (c) Name the element with the smallest atomic size
- (d) Name the element which is a metalloid
- (e) Name the element that shows maximum valency
Answer:
- (a) The most electropositive element among them is Lithium
- (b) The most electronegative element among them is Fluorine
- (c) The element with the smallest atomic size is Fluorine
- (d) Boron (5) is a metalloid
- (e) Carbon shows maximum valency
Question8.
An element X, a yellow solid at room temperature, shows catenation and allotropy. X forms two oxides formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants?
Year of Question :(2016)
- (a) Identify the element X
- (b) Write the electronic configuration of X
- (c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals
- (d) What would be the nature (acidic/ basic) of oxides formed
- (e) Locate the position of the element in the Modem Periodic Table
Answer:
- (a) The element X is Sulphur
- (b) The electronic configuration of X is 2, 8, 6
- (c) Thermal decomposition of ferrous sulphate:
2 FeSO4 → Fe2O3 + SO2 + SO3
- (d) Oxides of Sulphur are acidic
- (e) Sulphur belongs to Group 16 and the third period of the periodic table
Question9.
An element X of group 15 exists as a diatomic molecule and combines with hydrogen at 773 K in the presence of the catalyst to form a compound, ammonia, which has a characteristic pungent smell?
Year of Question :(2017)
- (a) Identify the element X. How many valence electrons does it have
- (b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it
- (c) Draw the electron dot structure for ammonia, and what type of bond is formed in it
Answer:
- (a) The element X is Nitrogen. It has five valence electrons
- (b) The electron dot structure of the diatomic molecule of X.
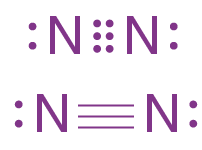 Dinitrogen forms a covalent bond
Dinitrogen forms a covalent bond
- (c ) The electron dot structure of the ammonia.
Ammonia forms a covalent bond
Question10.
Which group of elements could be placed in Mendeleevs Table without disturbing the original order? Give reason?
Year of Question :(2013)
Answer:
Noble gases could be placed in Mendeleev.s periodic table without disturbing the original order.
Noble gases are inert due to a wholly filled valence electron shell. They are present in low concentrations in our atmosphere and do not form any compound with other elements. During the evolution of the periodic table by Mendeleev, these gases were not known. After their discovery, they were placed in a separate group as their properties did not resemble that of any other group in the periodic table. So, their existence did not affect the existing order of Mendeleevs periodic table
Question11.
Give an account of the process adopted by Mendeleev for the classification of elements. How did he arrive at Periodic Law?
Year of Question :(2012)
Answer:
Mendeleev tried to classify the elements based on their chemical properties to ease the study of elements. He wrote the properties of each element on a separate card and arranged them in diverse ways. He put all 63 elements in the order of their increasing atomic masses in horizontal rows called periods. Elements with similar properties were placed below the other in the same vertical column called groups. There was a total of seven periods and eight groups. The classification was based on resemblances in physical and chemical properties and the compounds formed by elements with hydrogen and oxygen. He noticed that elements of similar properties would repeat at regular intervals (8th, 18th, or 32nd position). Then, he stated it as the periodic law, i.e. The properties of elements are a periodic function of their atomic masses
Important Questions and Answers from Chapter:Periodic Classification of Elements
Question 1:
What is Döbereiner’s Law of Triads?
Answer:
Johann Wolfgang Döbereiner (German scientist) introduced the "Law of Triads" in the early 19th century.
It states that when three elements with similar properties are arranged in increasing order of their "atomic weights", the atomic weight of the middle element is the average of the other two.
Example:
The triad of Lithium (Li), Sodium (Na), and Potassium (K):
Atomic weight of Sodium ≈ (Atomic weight of Lithium + Atomic weight of Potassium) ÷ 2.
Question 2:
What were the limitations of Döbereiner’s Triads?
Answer:
Only a few elements could be grouped into triads.
Several known elements didn’t fit into any triad.
Failed to classify all elements known at that time.
Question 3:
Explain Newlands’ Law of Octaves.
Answer:
John Newlands, a British scientist, proposed the "Law of Octaves" in 1864.
Main Points:
When elements are arranged in the order of "increasing atomic weights", the properties of every eighth element are similar to the first, like musical notes.
He compared it to musical octaves where every eighth note is similar.
Limitations:
The law worked only up to Calcium.
Newlands placed elements with different properties in the same group (e.g., Iron with Oxygen and Sulfur).
Question 4:
What is Mendeleev’s Periodic Law? What were its achievements?
Answer:
Mendeleev’s Periodic Law:
Dmitri Ivanovich Mendeleev, a Russian chemist, proposed this law in 1869.
The "properties of elements are a periodic function of their atomic masses".
Achievements:
Prediction of new elements: Mendeleev left gaps in his table for undiscovered elements and predicted their properties (e.g., Eka-aluminium was later discovered as Gallium).
Correction of atomic weights: Mendeleev corrected the "atomic weights" of some elements like Beryllium.
Grouping of similar elements: Elements with similar properties were grouped together, making their study easier.
Question 5:
What were the limitations of Mendeleev’s Periodic Table?
Answer:
Position of hydrogen: It could not be clearly assigned to a specific group because hydrogen behaves like both alkali metals and halogens.
No space for isotopes: Isotopes (same element with different atomic masses) were not given separate positions.
Irregular atomic mass: Some heavier elements were placed before lighter ones (e.g., Cobalt before Nickel).
Uncertainty for elements: In some cases, the increase in atomic weights between elements was not regular, making it hard to predict the number of elements between them.
Question 6:
Explain Moseley’s Modern Periodic Law.
Answer:
Henry Moseley, a British physicist, proposed the "Modern Periodic Law" in 1913.
It states that "the physical and chemical properties of elements are periodic functions of their atomic numbers".
Key Points:
Moseley used X-rays to study elements and found that "atomic number", not atomic weight, is the fundamental property.
This law led to the arrangement of elements by increasing "atomic number" in the "Modern Periodic Table".
Question 7:
What is the Modern Periodic Table? How is it structured?
Answer:
Structure:
Developed by Bohr and based on Moseley’s work.
Elements are arranged in increasing order of "atomic numbers".
It has 18 vertical columns called "groups" and 7 horizontal rows called "periods".
Key Features:
Elements in the same "group" have similar properties (e.g., all elements in Group 1 are alkali metals).
The table includes both naturally occurring elements (1 to 92) and synthetic elements (93 to 118).
Question 8:
What are the periodic properties of elements?
Answer:
Valency:
Valency refers to the number of electrons in the outermost shell.
Increases from 1 to 4 across a "period", then decreases to 0.
Atomic Size:
Atomic radius increases down a "group" (due to added shells).
Decreases across a "period" (due to increased nuclear charge pulling electrons closer).
Metallic and Non-metallic Properties:
Metals are on the left side of the periodic table and lose electrons to form positive ions.
Non-metals are on the right side and gain electrons to form negative ions.
Ionization Energy:
Energy required to remove an electron from an atom.
Decreases down a "group" and increases across a "period".
Electron Affinity:
Energy released when an atom gains an electron.
Increases across a "period", decreases down a "group".
Electronegativity:
Ability of an atom to attract shared electrons in a bond.
Increases across a "period", decreases down a "group".
Question 9:
How does atomic size change across a period and down a group?
Answer:
Across a period:
Atomic size decreases as the nuclear charge increases, pulling electrons closer to the nucleus.
Down a group:
Atomic size increases because new electron shells are added, increasing the distance between the nucleus and outermost electrons.
Question 10:
Why were inert gases placed in a separate group in the periodic table?
Answer:
Inert gases (e.g., Helium, Neon) have fully filled outermost electron shells.
They are "chemically unreactive" and do not readily form compounds.
A separate group (Group 18) was created for these gases in both Mendeleev’s and the modern periodic table.