NCERT Solutions for Class 10 Science Chapter 2 Acids Bases and Salts
Question1.
You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Answer:Take a small volume of all three liquids in three different test tubes. Dip red litmus paper strips separately in all the three test tubes. The tube in which red litmus strip turns blue, contains a basic solution. Now, we use the blue litmus paper as testing paper and dip it into the remaining two solutions. The solution which changes the colour of the blue litmus paper into red is acidic and the other which does not affect it, is neutral, i.e. distilled water
Question2.
Why should curd and other sour substances not be kept in containers made up of brass or copper?
Answer:Both curd and other sour substances contain some acids in them. They react with copper or brass vessels to form certain salts which are not good for health. Therefore, it is not advisable to keep them in brass or copper containers
Question3.
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Answer:Metals are mostly reactive in nature. They react with dilute acids (HCl and H2SO4) to evolve hydrogen gas. For example,
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
The gas burns with a pop sound when a burning candle is brought near to it
Question4.
A metallic compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride?
Answer:The gas evolved with effervescence and extinguishes a lit candle is CO2. If one of the compounds formed is CaCl2, the reaction would be
CaCO3(s) + HCl(aq) → CaCl2(aq) + CO2 + H2O(l)
Question5.
Aqueous solutions of HCl, HNO3 and H2SO4, etc. show acidic character while those of the compounds like ethyl alcohol (C2H5OH) and glucose (C6H12O6) fail to do so. Explain?
Answer:All the listed acids have replaceable hydrogen atoms which they release in aqueous solution as hydrogen ions. Therefore, they show acidic character. However, both ethyl alcohol (C2H5OH) and glucose (C6H12O6) do not undergo dissociation in aqueous solution. That why they do not conduct electricity in aqueous solution
Question6.
Why does an aqueous solution of an acid conduct electricity?
Answer:Aqueous solution of an acid releases H+ and H3O+ in solution. Since ions are carriers of charge, therefore they are responsible for conducting electricity
Question7.
Why does dry HCl gas not change the colour of dry litmus paper?
Answer:A dry HCl gas has no H+ so it does not show any acidic character therefore, no change in colour takes place until we moisten the litmus paper
Question8.
While diluting an acid, why is it recommended to add acid to water and not water to the acid?
Answer:Mineral acids such as, H2SO4, HNO3, HCl, etc. have strong affinity for water, so dilution of acid is highly exothermic in nature. This heat may cause jumping of solution or cracking of apparatus. In order to avoid it, acid is added drop by drop to water which dilutes the heat and prevent accident
Question9.
How is concentration of Hydronium ions (H3O+) affected when solution of an acid is diluted?
Answer:An acid dissociates into hydronium ions (H3O+) and anions when dissolved in water. Upon dilution, the volume of the solution increases and the number of ions per unit volume decreases. Therefore, the concentration of H3O+ per unit volume decreases
Question10.
How is concentration of hydroxyl (OH-) ions affected when excess of base is dissolved in solution of sodium hydroxide?
Answer:Sodium hydroxide (NaOH) is a strong base. It immediately dissociates in solution to give OH- and cat ions. Upon dissolving more of the base in the solution, the concentration of OH- further increases
Question11.
You have two solutions A and B. The pH of solution A is 6 and that of solution B is 8. Which solution has more hydrogen ion concentration? Which of these is acidic and which one is basic?
Answer:The pH of a solution is inversely proportional to the concentration of H+ in solution. Lesser the pH of the solution, more will be the H+ concentration. The solution A with pH 6 has more H+ concentration than the solution with pH equal to 8. The solution A is acidic because its pH is less than 7 and the solution B is basic because its pH is more than 7
Question12.
What effect does concentration of H+(aq) have on acidic nature of a solution?
Answer:The acidic nature of a solution is directly related to the concentration of H+. As the concentration of H+ increases, the acidic nature of the solution also increases
Question13.
Do basic solutions also have H+(aq)? If yes, then why are these basic?
Answer:Yes, basic solutions also have H+. As the solutions are prepared in water and water being a weak electrolyte, it dissociates into H+ and OH- but the number of H+ are very small as compared to OH- ions
Question14.
Under what soil conditions, do you think a farmer would spread or treat the soil of his field with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Answer:A soil usually becomes acidic when there is either a high peat content or, iron minerals or there are some rotten vegetables in the soil. In order to reduce the acidic strength, liming of soil is usually done. For this, any of the substances that have been mentioned are added to the soil since they are of basic nature
Question15.
Name the substance which upon treating with chlorine gives bleaching powder. Write the chemical equation for the reaction?
Answer:Slaked lime is the substance which reacts with chlorine to give bleaching powder
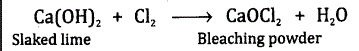
Question16.
Name the sodium compound used for softening hard water?
Answer:Washing soda or sodium carbonate decahydrate
(Na2CO3.10H2O)
Question17.
What will happen if the solution of sodium hydrogen carbonate is heated? Write the chemical equation involved?
Answer:Carbon dioxide gas will evolve and sodium car-bonate will be formed.
2NaHCO3 → Na2CO3 + CO2 + H2O
Question18.
Write the chemical equation for the reaction between Plaster of Paris and water?
Answer:

Question1.
What is the common name of the compound CaOCl2?
Answer:The common name of the compound CaOCl2 is bleaching powder
Chapter End Questions
Question1.
A solution turns red litmus blue. Its pH is likely to be?
Answer:
Question2.
A solution reacts with crushed egg-shells to give a gas that turns lime water milky. The solution contains?
Answer:
Question3.
10 mL of solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise will be?
Answer:
Question4.
Which of the following types of medicines is used for treating indigestion?
- Antibiotic
- Analgesic
- Antacid
- Antiseptic
Answer:
Question5.
Write the word equation and the balanced equations for the reactions when?
- dilute sulphuric acid reacts with zinc granules
- dilute hydrochloric acid reacts with magnesium ribbon
- dilute sulphuric acid reacts with aluminium powder
- dilute hydrochloric acid reacts with iron filings
Answer:
- Word equation:
Zinc + Sulphuric add →Zinc sulphate + Hydrogen
Balanced equation:
Zn(s) + H2SO4(dil.) → ZnSO4(aq) + H2(g)
- Word equation:
Magnesium + Hydrochloric acid → Magnesium chloride + Hydrogen
Balanced equation:
Mg(s) + 2HCl (dil.) → MgCl2(aq) + H2(g)
- Word equation:
Aluminium + Sulphuric acid →Aluminium sulphate + Hydrogen
Balanced equation:
2Al(s) + 3H2SO4(dil.) → Al2(SO4)3(aq) + 3H2(g)
- Word equation:
Iron + Hydrochloric acid → Iron chloride + Hydrogen
Balanced equation:
Fe(s) + 2HCl(dil.) → FeCl2(aq) + H2(g)
Question6.
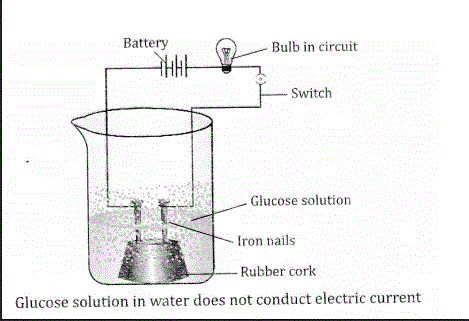
Compounds such as alcohol and glucose also contain hydrogen but are not characterised as acids. Describe an activity to prove it?
Answer:Compounds such as alcohol and glucose also contain hydrogen but do not behave like an acid. Both are organic compounds with the formulae C2H5OH and C6H12O6, respectively. This can be proved by the following activity:
In a glass beaker, take a dilute solution of glucose (C6H12O6). Fix two small nails of iron in a rubber cork and place the cork in the beaker as shown in the figure. Connect the nails to the terminals of a 6 volt battery through a bulb. Switch on the current. The bulb will not glow. This shows that the electric current has not passed through the glucose solution. As the current is carried by the movement of ions, it shows that the solution of glucose has not given any H+. Now repeat the same experiment with ethyl alcohol (C2H5OH). The bulb will again not glow.
This shows that both of them do not behave as acids although they contain hydrogen atoms in their molecules
Question7.
Why does distilled water not conduct electricity whereas rain water does?
Answer:Pure or distilled water has no ions as it is a very weak electrolyte. So, no conduction of electricity takes place but rain water contains dissolved acids and so rain water is a good conductor of electricity
Question8.
Why does an acid not show any acidic behaviour in the absence of water?
Answer:The acidic behaviour of a substance is due to the presence of H+(aq) ions. As acids do not dissociate to produce H+(aq) ions in the absence of water so they do not show acidic behavior
Question9.
Five solutions A, B, C, D and E when tested with universal indicator show pH as 4, 1, 11, 7 and 9, respectively. Which solution is?
- neutral
- strongly alkaline
- strongly acidic
- weakly alkaline
- weakly acidic
Arrange the pH in increasing order of H+ concentration.
Answer:
- Neutral: D with pH = 7
- Strongly alkaline: C with pH = 11
- Strongly acidic: B with pH = 1
- Weakly alkaline: E with pH = 9
- Weakly acidic: A with pH = 4
Increasing order of H+ concentration:
C<E<D<A<B
Question10.
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A while acetic acid (CH3COOH) is added to test tube B. In which case, fizzing occurs more vigorously and why? Fizzing in the reaction is due to the evolution of hydrogen gas by the action of metal on the acid?
- Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
- Mg(s) + 2CH3COOH(aq) → (CH3COO)2Mg(aq) + H2(g)
Since hydrochloric acid is a stronger acid than acetic acid, fizzing occurs more readily in tube A than in tube B. Actually hydrogen gas will evolve at more brisk speed in test tube A.
Question11.
Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer?
Answer:When milk changes into curd, the pH decreases. Lactose (carbohydrate) present in milk gets converted into lactic acid. As more acid is formed, pH of the medium decreases
Question12.
A milkman adds a very small amount of baking soda to fresh milk?
- Why does he shift the pH of the fresh milk from 6 to slightly alkaline
- Why does milk take a long time to set as curd
Answer:
- Fresh milk is slightly acidic due to the presence of lactic acid. The presence of bacteria decreases the pH of milk and makes it sour. To prevent it, baking soda (NaHCO3) is added to neutralise the acidic nature making it slightly alkaline
- When milk changes to curd, it becomes more acidic but adding baking soda neutralises it and checks curdling
Question13.
Plaster of Paris should be stored in a moisture-proof container. Why?
Answer:The presence of moisture can affect the slow setting of plaster of Paris by bringing about its hydration. This will make the plaster of Paris useless after some time.
Question14.
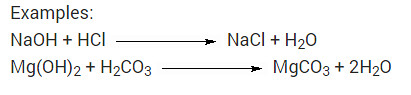
What is neutralisation reaction? Give two examples?
Answer:The reaction of an acid and a base, giving rise to the corresponding salt and water is called neutralization reaction.
Examples:
NaOH + HCl → NaCl + H2O
Mg(OH)2 + H2CO3 → MgCO3 + 2H2O
Question15.
Give two important uses of washing soda and baking soda?
Answer:Washing soda
1. It is often used as an electrolyte.
2. Domestically it is used as a water softener during laundry.
Baking soda
1. It is used to test garden soil for acidity. If it develops bubbles, the soil is too acidic.
2. Washing a car with it can remove dead bug bodies without damaging the paint.
NCERT Solutions for Class 10 Science Chapter 2 (MCQs) [1 Mark each]
Question 1.
Equal pieces of zinc granules are dropped in four test tubes. Following substances are poured in all the four test tubes. The reaction will be vigorous with [CCE 2014]
(a) CH
3COOH
(b) HCl
(c) sodium bicarbonate solution
(d) lemon juice
Answer:
(b) Strong adds like HCl react vigorously with active metals like Zn and form metal salt and evolve H
2 gas.
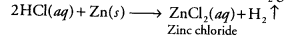
Question 2.
Which of the following statements shows the property of an acid? [CCE 2014]
(a) It turns blue litmus to red
(b) It is sour in taste
(c) It has no effect on red litmus
(d) All of the above
Answer:
(d) An acid turns blue litmus red. Thus, it has no effect on red litmus and acids are sour in taste.
Question 3.
A drop of a liquid sample was put on the pH paper. It was observed that the colour of the pH paper turned blue. The liquid sample is [CCE 2014]
(a) lemon juice
(b) sodium bicarbonate solution
(c) distilled water
(d) hydrochloric acid
Answer:
(b) The liquid sample is of sodium bicarbonate (NaHC
3) solution. It is a basic solution. And we know that a basic solution turns pH paper blue.
Question 4.
Two solutions X and Y were found to have pH value of 4 and 10 respectively. The inference that can be drawn is [CCE 2014]
(a) X is a base and Y is an acid
(b) Both X and Y are acidic solutions
(c) X is an acid and Yis a base
(d) Both X and Y are bases
Answer:
(c) Any solution having pH > 7 will be a base while the solution having pH < 7 will surely be an acid. Hence, it can be concluded that X is an acid (pH=4, i.e. < 7) and Yis a base (pH =10, i.e. > 7).
Question 5.
A student was asked to collect apparatus from lab store, for doing experiment of pH of given sample. Identify the article which he is not supposed to pick. [CCE 2014]
(a) pH paper
(b) Dropper
(c) Litmus paper
(d) Petri dish
Answer:
(d) Petri dish is not required for doing experiment of pH.
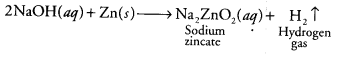
Question 6.
Which one of the following would you need to identify the gas that evolve when you heat NaOH solution with zinc metal? [CCE 2014]
(a) Red litmus solution
(b) Blue litmus solution
(c) A burning splinter / matchstick
(d) Lime water
Answer:
(c) When a base like NaOH is treated with any active metal like Zn, it produces H2 gas. And the presence of the hydrogen gas can be tested by bringing a burning splinter/ matchstick near the gas produced. The gas will burn with a pop sound confirming the presence of hydrogen gas.